Solid – Liquid – Gas: An Integrated S.T.E.M. Lesson
Overview
Our first lesson was an opportunity to take the S.T.E.M. lab outdoors and launch our model rockets while studying forces in motion. This time we’ll be heading back to the lab working with the states of matter, chemical changes, and physical changes. This project promises to be a fun activity, appropriate for grades 3-5, at a minimal cost.
By the completion of this lesson, your students will be able to differentiate between a chemical change and a physical change as well as identify the properties of solids, liquids and gases.
For this project, you will need:
- a set of small funnels, baking soda
- white vinegar
- empty 1-liter soda bottles
- balloons
- kitchen measuring scoops
You will want to cover your lab tables with some plastic sheeting to make cleaning up any accidental spills a simple task.
Begin by holding a discussion with your class on the states of matter. Discover what they already know and build upon it. A solid has a definite size and shape because the molecules are held so tightly together. A liquid has a definite volume and can take the shape of its container and spill out onto the floor when let out of its container because its molecules are looser. A gas will also take the shape of its container but has no definite volume as its molecules are bouncing around even more loosely. Ask students to point out examples of solids, liquids, and gases in their classroom or in their daily lives.
Next examine some of the materials to be used in the experiment. The students should be able to recognize that the white vinegar is in a liquid state. It may be confusing to some that the baking soda, although in powder form, is in fact a solid. Use a magnifying glass to have your class inspect the solid granules under magnification.
So, you have a solid and a liquid, but where is the gas? Here comes the interesting part. We are going to use the solid and the liquid to create the gas. First measure and pour 1 cup of the vinegar into the empty soda bottle. Then, gently roll a balloon onto the end of the funnel. While gripping it tightly to the funnel, scoop 1/3 of a cup of baking soda into the funnel. Do this a little at a time and let the baking soda pack into the balloon. Now attach the balloon over the top of the soda bottle. Be careful as to avoid ripping the balloon or tilt it upwards – at least not yet.
Pause your experiment at this point to have a discussion about the difference between a physical change and a chemical change. The result of a physical change is that the substance will have a different appearance, whereas a chemical change produces new substances. Examples of physical changes are melting ice, freezing water, tearing paper, etc. – any situation where the original substance’s appearance is changed. Examples of chemical change are burning of wood and rusting of metal – any result from a chemical reaction in which a new substance or substances are formed.
And now, back to the experiment. Tilt the balloon upright so that the baking soda falls into the bottle and contacts the vinegar. Hold onto the bottle and pay close attention to what is taking place inside the bottle. There will be a lot of bubbling and fizzing and the balloon will begin to inflate. Congratulations! You have just used a solid and a liquid to create a gas. The gas released from the chemical reaction of the baking soda and vinegar is now trapped inside of the balloon.
Science
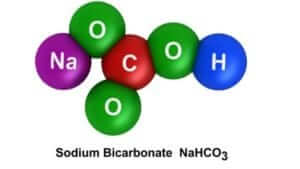
So, what is the science behind the balloon inflation? The acid from the vinegar breaks down the baking soda and releases carbon dioxide. The escaping gas fills the soda bottle and is trapped in the balloon. That was your chemical change. The chemical reaction produced a new substance (CO2 gas). This lesson also incorporated the identification of the properties of the states of matter.
Technology
The technology behind this project is the ability to inflate a balloon without ever applying our lips to it or applying the force of the air from our lungs. We accomplish this by capturing the gas released by a chemical reaction. Also, we are using an acid to break the bonds in the baking soda in order to release the CO2.
Engineering
The engineering aspect included the method required to pack the baking soda into the balloon and connect the balloon to the bottle. We used a funnel because directly scooping the baking soda into the balloon would have been difficult. The balloon itself is used in a non-traditional way. It acts as the container for the baking soda as well as the delivery system and the collection receptacle for the gas.
Mathematics
The math lies in making the proper measurements of the baking soda and vinegar. Students can visualize cups, ounces, tablespoons, etc. – which are typical units used in their mathematics classes, but perhaps rarely used in an actual setting.
Extend-Expand-Explore
Extend the lesson to explore variations in the quantities of baking soda and vinegar. Expand on the mathematics by converting standard measurements in metrics. Experiment by changing the quantities of these two variables to see if you can produce more or less gas.
Lesson Plan
Title: Solid-Liquid-Gas
Objectives: To identify the properties of solids, liquids, and gases and to differentiate between a chemical and physical change.
Materials:
- baking soda
- funnels
- balloons
- white vinegar
- kitchen measuring scoops
Procedure:
- Measure and pour 1 cup of the vinegar into the empty soda bottle.
- Roll a balloon onto the end of the funnel.
- Scoop 1/3 cup baking soda into the funnel.
- Attach the balloon over the top of the soda bottle.
- Tilt the balloon upright so that the baking soda falls into the bottle.
- The balloon will begin to inflate.
Conclusion:
The chemical reaction between the vinegar (liquid) and baking soda (solid) released carbon dioxide (gas) which was captured in the balloon.

- Christopher Masullo, Ed.D.





